Facile synthesis of branched polyvinyl acetate via redox-initiated radical polymerization†
Abstract
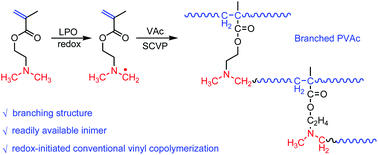
Although branched polymers find widespread applications, the rational design and synthesis of branched vinyl polymers via the conventional radical (co)polymerization of commercially available monomers is still a challenge for researchers in this field. Using branched polyvinyl acetate (PVAc) as a proof-of-concept, we report a facile approach to synthesize branched polymers via redox-initiated radical copolymerization. In this case, the commercially available monomer 2-(dimethylamino)ethyl methacrylate (DMAEMA), containing both a vinyl group and an initiator fragment (tertiary amine group), serves as an inimer in the presence of an oxidant to copolymerize with vinyl acetate (VAc), leading to the formation of branching architectures. The copolymerization kinetics investigation as well as electron paramagnetic resonance (EPR) measurements were conducted to reveal the reactivity of DMAEMA and the development of branching architectures, while TD-SEC and 1H-NMR were employed to study the structures of the resulted polymers. The results confirm that DMAEMA can serve as an inimer for the preparation of branched PVAc. Moreover, balancing the feed ratio of DMAEMA to VAc enables the fine control of branching degree. Within our experimental design, a high feed ratio of DMAEMA to VAc leads to high branching, as demonstrated by the Zimm branching factor, g′. This novel methodology involves only commercially available monomers and conventional radical polymerization and hence offers a promising future for preparing branched vinyl polymers on a large scale and at low cost.


 Please wait while we load your content...
Please wait while we load your content...