Origins of the enantioselectivity of a palladium catalyst with BINOL–phosphoric acid ligands†
Abstract
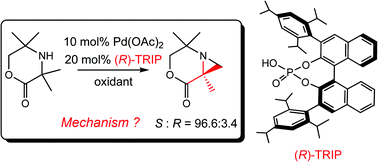
Transition metal-catalyzed C–H activation with high enantioselectivity is a new and challenging field. BINOL–phosphoric acid ligands ((R)-TRIP) were found to be able to induce high enantioselectivity in aziridination of aliphatic amines by palladium-catalysis, and its origins are investigated in this study. We unveiled that in the effective catalyst, it is the acetate ligand rather than the phosphate group that participates in assisting palladium catalysis due to its weaker Brønsted acidity. By comparison with two other modified ligands, we demonstrate that the isopropyl groups of (R)-TRIP provide it with extra degrees of freedom to affect the transition states leading to the S and R aziridination products in different ways. They create a roomy space for the acetate in the S transition state but cause a steric repulsion for it in the R transition state, making the former pathway much more favorable and achieving the enantioselectivity. This determination occurs entirely within the catalyst. This study deepens our understanding of transition metal-catalysis and provides new insights into the rational design of catalysts of high enantioselectivity.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...