Synthesis of highly rigid phosphine–oxazoline ligands for palladium-catalyzed asymmetric allylic alkylation†
Abstract
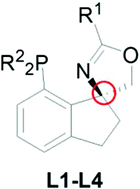
A highly rigid spiro phosphine–oxazoline ligand skeleton with a spirocarbon stereogenic center was developed from 7-bromo-1-indanone. The catalytic performance of the ligand was demonstrated in palladium-catalyzed asymmetric allylic alkylation. Under optimized conditions, high yields (up to 99%) and enantioselectivities (up to 99.9% ee) were obtained for reactions of 1,3-diphenylallyl acetates and symmetrical 1,3-dicarbonyl substrates.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...