Palladium(ii)-mediated rapid 11C-cyanation of (hetero)arylborons†
Abstract
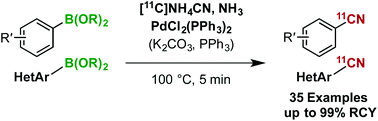
A palladium(II)-mediated rapid 11C-cyanation of (hetero)arylborons with [11C]NH4CN/NH3 has been developed using bench-stable and readily available reagents. The method showed excellent functional-group tolerance, and allowed the highly efficient synthesis of a wide range of [11C]cyanoarenes, including PET tracers for aromatase imaging. A mechanistic study of the 11C-cyanation suggests the instantaneous formation of a mono[11C]cyanopalladium(II) complex that reacts smoothly with arylborons.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...