Synthesis and anticholinesterase activity of 2-substituted-N-alkynylindoles†
Abstract
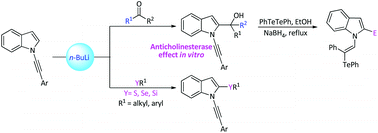
In this paper, we report a protocol for the preparation of 2-substituted-N-alkynylindoles via metalation of N-alkynylindoles followed by the capture of a 2-indolyl lithium intermediate with different electrophiles. The reactivity of the indoles prepared was also demonstrated through the reaction with CBr4/Ph3P for the preparation of 2-gem-dibromovinyl N-alkynylindoles and the hydrotelluration reaction of N-alkynylindoles, which led to vinylic tellurides. Some compounds prepared showed AChE inhibitory potential in the low micromolar range similar to that obtained with donepezil, a commercially available cholinesterase inhibitor.


 Please wait while we load your content...
Please wait while we load your content...