Electrophilic halogenation of hydrazones of CF3-ynones. Regioselective synthesis of 4-halo-substituted 3-CF3-pyrazoles†
Abstract
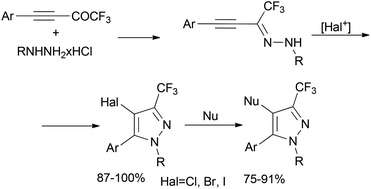
An efficient approach for the synthesis of 4-halo-3-CF3-pyrazoles was elaborated using the cyclization of hydrazones of CF3-ynones induced by electrophilic halogenation. This method can be used for the regioselective synthesis of 3-CF3-pyrazoles substituted at the 4-position with any halogen (I, Br or Cl). The high (up to quantitative) yields, mild conditions and simplicity of the reaction protocol are the advantages of the proposed method. It was demonstrated that cross-coupling with 4-iodo-3-CF3-pyrazoles can be used for the synthesis of a broad family of tetrasubstituted pyrazoles. Moreover, it was found that this method is suitable for the synthesis of 4-substituted derivatives of Celebrex and SC-560.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...