Modular synthesis of (E)-cinnamaldehydes directly from allylarenes via a metal-free DDQ-mediated oxidative process†
Abstract
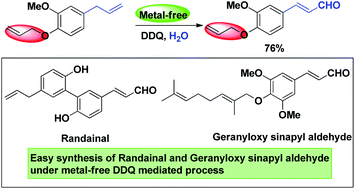
An efficient synthesis of (E)-cinnamaldehydes by a metal-free DDQ-mediated oxidative transformation of allylarenes was developed. The protocol provides a practical method to prepare diverse (E)-cinnamaldehydes with broad functional group tolerance in good to excellent yields, including easy access to natural products randainal and geranyloxy sinapyl aldehyde from plant extracts. Finally, the mechanism of a single-electron transfer process was proposed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...