Nα-Amino acid containing privileged structures: design, synthesis and use in solid-phase peptide synthesis†
Abstract
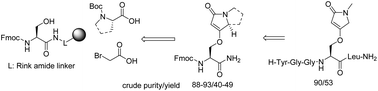
Fmoc-protected Nα-amino acid containing heterocyclic privileged structures, O-(1-methyl-5-oxo-2,5-dihydro-1H-pyrrol-3-yl)-L-serine and O-((S)-5-oxo-2,3,5,7a-tetrahydro-1H-pyrrolizin-7-yl)-L-serine, were synthesized on the solid phase from simple commercially available building blocks under mild conditions. The amino acid side-chain is composed of tetramic acid, a natural product derived privileged structure. The key transformation was the formation of cyclic enol ethers via nonclassical Wittig olefinations of the esters. Solid-phase synthesis represents a method of choice, particularly for the synthesis of peptides. This route is compatible with traditional Merrifield solid-phase peptide synthesis (SPPS), as documented on the preparation of the pentapeptide Leu-enkephalin amide H-Tyr-Gly-Gly-Phe-Leu-NH2 with Phe or Tyr replaced by a novel amino acid.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...