Base-promoted diastereoselective α-alkylation of borane N-((S)-1′-phenylethyl)azetidine-2-carboxylic acid ester complexes†
Abstract
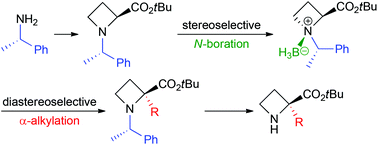
The base-promoted α-alkylation of N-((S)-1-phenylethyl)azetidine-2-carboxylic acid esters 1 was investigated. The use of diastereomerically pure borane complexes 3 as substrates, which are easily prepared from 1, dramatically improved the yields and diastereoselectivities of α-alkylated products 2. For example, the treatment of tert-butyl ester (1S,2S,1′S)-3a with 2.4 equivalents of lithium bis(trimethysilyl)amide (LiHMDS) at 0 °C followed by 2.6 equivalents of benzyl bromide afforded α-benzylated (2S,1′S)-2aa in 90% yield as almost a single diastereomer. Our method enables the production of optically active α-substituted azetidine-2-carboxylic acid esters starting from commercially available (S)-1-phenylethylamine, which is one of the least expensive chiral compounds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...