Phenolate-induced intramolecular ring-opening cyclization of N-tosylaziridines: access to functionalized benzoxacycles†
Abstract
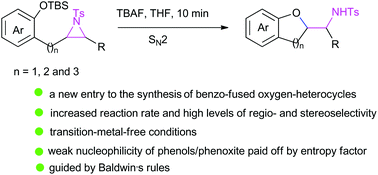
Phenolate-induced, diastereo- and regioselective intramolecular exo-tet ring-opening cyclization of N-tosylaziridines has been achieved for the first time. The N-tosylaziridine substrates bearing a tethered (ortho-(tert-butyldimethylsiloxy))aryl substituent, prepared directly from the corresponding olefins under Sharpless aziridination conditions, furnished functionalized 2,3-dihydrobenzofuran, chroman, and 1-benzoxepane derivatives in excellent yields when treated with tetrabutylammonium fluoride (TBAF) at room temperature. Our ability to synthesize benzoxacycle-based N-tosyl-protected amino alcohols, that are otherwise difficult to obtain by traditional synthetic routes, has opened the door to diversify the chemistry of β-amino alcohols. We also succesfully performed the Baeyer–Villiger oxidation of a salicylaldehyde ether bearing a tethered N-tosylaziridine moiety with m-CPBA followed by tandem saponification and 6-exo-tet aziridine ring-opening cyclization, furnishing the corresponding trans-3,4-disubstituted-1,4-benzodioxane derivative. Overall, the study has unveiled a new entry to the synthesis of benzoxacycles and has also broadened the impact of aziridines as synthetic building blocks.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...