Synthesis of N-alkylated 2-pyridones through Pummerer type reactions of activated sulfoxides and 2-fluoropyridine derivatives†
Abstract
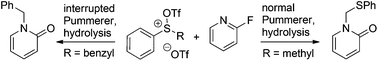
N-Alkylated 2-pyridone products were obtained in good to excellent yields through a one-pot procedure involving either normal or interrupted Pummerer reactions between triflic anhydride activated sulfoxides and 2-fluoropyridine derivatives, followed by hydrolysis. This is a rare case that uses 2-fluoropyridine as a nucleophile in Pummerer type reactions.


 Please wait while we load your content...
Please wait while we load your content...