Chemoenzymatic synthesis of cytokinins from nucleosides: ribose as a blocking group†
Abstract
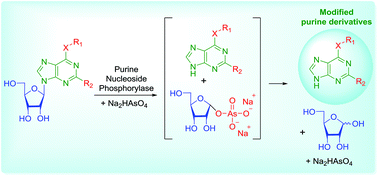
Nucleoside phosphorylases are involved in the salvage pathways of nucleoside biosynthesis and catalyze the reversible reaction of a nucleobase with α-D-ribose-1-phosphate to yield a corresponding nucleoside and an inorganic phosphate. The equilibrium of these reactions is shifted towards nucleosides, especially in the case of purines. Purine nucleoside phosphorylase (PNP, EC 2.4.2.1) is widely used in labs and industry for the synthesis of nucleosides of practical importance. Bacterial PNPs have relatively broad substrate specificity utilizing a wide range of purines with different substituents to form the corresponding nucleosides. To shift the reaction in the opposite direction we have used arsenolysis instead of phosphorolysis. This reaction is irreversible due to the hydrolysis of the resulting α-D-ribose-1-arsenate. As a result, heterocyclic bases are formed in quantitative yields and can be easily isolated. We have developed a novel method for the preparation of cytokinins based on the enzymatic cleavage of the N-glycosidic bond of N6-substituted adenosines in the presence of PNP and Na2HAsO4. According to the HPLC analysis the conversion proceeds in quantitative yields. In the proposed strategy the ribose residue acts as a protective group. No contamination of the final products with AsO43− has been detected via HPLC-HRMS; simple analytical arsenate detection via ESI-MS has been proposed.


 Please wait while we load your content...
Please wait while we load your content...