One-step pathway to selenoisobenzofuran-1(3H)-imine derivatives through highly selective selenocyclization of olefinic amides with benzeneselenyl chloride†
Abstract
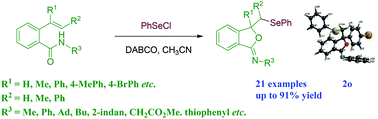
A 1,4-diazabicyclo[2.2.2]octane (DABCO) catalyzed selenocyclization of olefinic amides was achieved under mild reaction conditions. The reaction formed various benzeneselenyl substituted isobenzofuran-1(3H)-imine derivatives in good yields. The product was determined using single-crystal X-ray analysis. For compound 2u, the relative stereochemistry was established on the basis of NOESY NMR studies.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...