Synthesis and characterization of a photocleavable collagen-like peptide†
Abstract
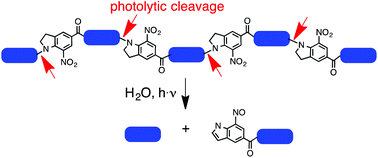
A 34-amino acid long collagen-like peptide rich in proline, hydroxyproline, and glycine, and with four photoreactive N-acyl-7-nitroindoline units incorporated into the peptide backbone was synthesized by on-resin fragment condensation. Its circular dichroism supports a stable triple helix structure. The built-in photochemical function enables the decomposition of the peptide into small peptide fragments by illumination with UV light of 350 nm in aqueous solution. Illumination of a thin film of the peptide, or a thin film of a photoreactive amino acid model compound containing a 5-bromo-7-nitroindoline moiety, with femtosecond laser light at 710 nm allows for the creation of well-resolved micropatterns. The cytocompatibility of the peptide was demonstrated using human mesenchymal stem cells and mouse embryonic fibroblasts. Our data show that the full-length peptide is cytocompatible as it can support cell growth and maintain cell viability. In contrast, the small peptide fragments created by photolysis are somewhat cytotoxic and therefore less cytocompatible. These data suggest that biomimetic collagen-like photoreactive peptides could potentially be used for growing cells in 2D micropatterns based on patterns generated by photolysis prior to cell growth.


 Please wait while we load your content...
Please wait while we load your content...