Cobalt and nickel bimetallic sulfide nanoparticles immobilized on montmorillonite demonstrating peroxidase-like activity for H2O2 detection†
Abstract
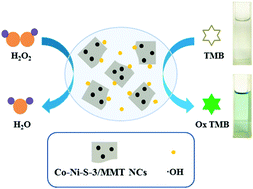
In this study, ternary transition metal sulfide (cobalt and nickel sulfides) nanoparticles were anchored on the surface of montmorillonite (MMT) by a facile one-pot hydrothermal method. These nanocomposites were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), and energy dispersive X-ray spectroscopy (EDS). Interestingly, for the first time, the obtained nanocomposites were found to possess intrinsic peroxidase-like activity, and they could rapidly catalyze the oxidation of the substrate 3,3′,5,5′-tetramethylbenzidine (TMB) into blue oxTMB in 3 min; also, clear color change could be quantified by absorbance at 652 nm using a UV-vis spectrophotometer. Studies showed that different proportions of cobalt sulfides and nickel sulfides in the nanocomposites affected the catalytic level of the intrinsic peroxidase-like activity. Co–Ni–S-3/MMT nanocomposites (NCs) exhibited the highest peroxidase-like activity compared with Co–S/MMT NCs and Ni–S/MMT NCs, suggesting that ternary transition metal sulfides (cobalt and nickel sulfides) possessed enhanced peroxidase-like activity compared with binary transition metal sulfides (cobalt sulfides or nickel sulfides). Similar to natural enzymes, Co–Ni–S-3/MMT NCs, as a peroxidase mimic, followed the typical Michaelis–Menten kinetics well and exhibited good affinity towards H2O2 and TMB. Moreover, the detection limit of H2O2 was determined to be as low as 1.5 μM, and the linear range was from 3 to 100 μM. Based on the peroxidase-like activity of the nanocomposites, a sensitive colorimetric sensor was designed and conveniently used for the detection of H2O2 in a contact lens solution.


 Please wait while we load your content...
Please wait while we load your content...