1-Ethyl-3-methylimidazolium tartrate chiral ionic liquids: preparation, characterization and opportunities thereof†
Abstract
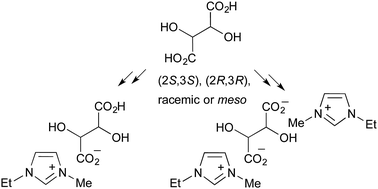
A unified acid/base synthetic access to tartrate-based chiral ionic liquids relying on the generation of cation hydroxide salts with AgOH was challenged with the preparation of sensitive 1-ethyl-3-methylimidazolium derivatives. Systematic variation of the starting tartaric acid stoichiometry and configuration led to eight stereoisomeric 1-ethyl-3-methylimidazolium hydrogen tartrate or di-(1-ethyl-3-methylimidazolium)tartrate entities. These salts were all characterised as proper ionic liquids. An unprecedented influence of the configuration ((2S,3S), or (2R,3R) vs. racemic or meso) on dynamic viscosity was observed. The relevance of such tartrate salts as task-specific ionic liquids was demonstrated in a synthetically useful base-promoted intramolecular cyclisation of a C2-symmetrical bis-epoxide en route to the total synthesis of phytofuran metabolites.


 Please wait while we load your content...
Please wait while we load your content...