Selected aryl thiosemicarbazones as a new class of multi-targeted monoamine oxidase inhibitors†
Abstract
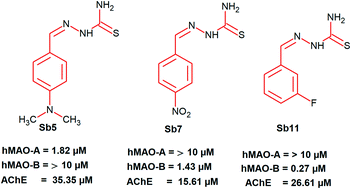
A series of 13 phenyl substituted thiosemicarbazones (SB1–SB13) were synthesized and evaluated for their inhibitory potential towards human recombinant monoamine oxidase A and B (MAO-A and MAO-B, respectively) and acetylcholinesterase. The solid state structure of SB4 was ascertained by the single X-ray diffraction technique. Compounds SB5 and SB11 were potent for MAO-A (IC50 1.82 ± 0.14) and MAO-B (IC50 0.27 ± 0.015 μM), respectively. Furthermore, SB11 showed a high selectivity index (SI > 37.0) for MAO-B. The effects of fluorine orientation revealed that SB11 (m-fluorine) showed 28.2 times higher inhibitory activity than SB12 (o-fluorine) against MAO-B. Furthermore, inhibitions by SB5 and SB11 against MAO-A and MAO-B, respectively, were recovered to near reference levels in reversibility experiments. Both SB5 and SB11 showed competitive inhibition modes, with Ki values of 0.97 ± 0.042 and 0.12 ± 0.006 μM, respectively. These results indicate that SB5 and SB11 are selective, reversible and competitive inhibitors of MAO-A and MAO-B, respectively. Compounds SB5, SB7 and SB11 showed moderate inhibition against acetylcholinesterase with IC50 values of 35.35 ± 0.47, 15.61 ± 0.057 and 26.61 ± 0.338 μM, respectively. Blood–brain barrier (BBB) permeation was studied using the parallel artificial membrane permeation assay (PAMPA) method. Molecular docking studies were carried out using AutoDock 4.2.


 Please wait while we load your content...
Please wait while we load your content...