Search for a 5-CT alternative. In vitro and in vivo evaluation of novel pharmacological tools: 3-(1-alkyl-1H-imidazol-5-yl)-1H-indole-5-carboxamides, low-basicity 5-HT7 receptor agonists†
Abstract
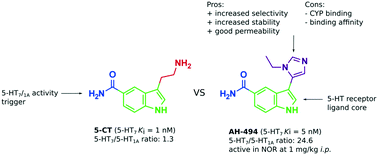
Close structural analogues of 5-carboxamidotryptamine (5-CT) based on the newly discovered indole–imidazole scaffold were synthesized and evaluated to search for a 5-HT7 receptor agonist of higher selectivity. In vitro drug-likeness studies and in vivo pharmacological evaluation of potent and selective low-basicity 5-HT7 receptor agonists, previously published 7 (3-(1-ethyl-1H-imidazol-5-yl)-1H-indole-5-carboxamide, AH-494) and 13 (3-(1-methyl-1H-imidazol-5-yl)-1H-indole-5-carboxamide), have supported their usefulness as pharmacological tools. Comprehensive in vitro comparison studies between 7, 13 and the commonly used 5-CT showed their very similar ADMET properties. Compound 7 at 1 mg kg−1 reversed MK-801-induced disruption in novel object recognition in mice and alleviated stress-induced hyperthermia (SIH) at high doses. Taking into account both in vitro and in vivo data, 7 and 13 may be considered as alternatives to 5-CT as pharmacological tools with important additional benefit associated with their low-basicity: high selectivity over 5-HT1AR.


 Please wait while we load your content...
Please wait while we load your content...