Efficient and selective oxidation of toluene to benzaldehyde on manganese tungstate nanobars: a noble metal-free approach†
Abstract
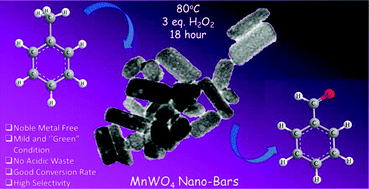
Selective production of benzaldehyde, an industrially relevant precursor material, by oxidation of the organic pollutant toluene, under mild environment-friendly green conditions, is still a challenging task. Herein, manganese tungstate (MnWO4) is demonstrated as an efficient catalyst to prepare benzaldehyde by direct oxidation of toluene in the presence of H2O2 as an oxidant at 80 °C via C–H activation, with an excellent conversion rate and high selectivity. MnWO4 nanobars (NBs) are synthesized via a single-step facile hydrothermal process and their catalytic performance for toluene oxidation is compared with that of MnWO4 nanoflakes (NFs) obtained by a precipitation method. Under the optimal conditions, a toluene conversion rate of 59.5% with 90% selectivity towards benzaldehyde has been achieved by using MnWO4 NBs, which is higher than that by MnWO4 NFs. This is due to a larger number of positively charged sites and an abundance of acidic sites on the NB as compared to the NF. Different reaction parameters such as reaction duration, oxidant concentration, catalyst concentration, and reaction temperature are varied systematically to obtain the optimal reaction conditions. The stability, recyclability, and leach test show no significant loss of catalytic activity and/or degradation of the catalyst. This proves the pure heterogeneity and recyclability of the catalyst, the important criteria for industrial applications.


 Please wait while we load your content...
Please wait while we load your content...