Configurationally flexible zinc complexes as catalysts for rac-lactide polymerisation†
Abstract
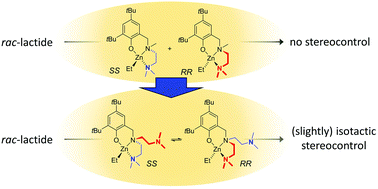
Zn(N(SiMe3)2)2 was reacted with pyridinemethanol and R,R–N,N′-di(methylbenzyl)-2,5-diiminopyrrole (L1H) to afford the dimeric complex (L1)2Zn2(μ-OR)2. The complex showed moderate activity in rac-lactide polymerization to heterotactic polymer (Pr = 0.75). 2,4-Di-tert-butyl-6-aminomethyl-phenol ligands with amino = N,N,N′,N′-tetramethyldiethylenetriamine (L2H) or di-(2-picoly)amine (L3H) were reacted with ZnEt2 to form (L2)ZnEt and with Zn(N(SiMe3)2)2 to form the respective amide complexes. All complexes, including (L1)2Zn2(μ-OR)2 were characterised by X-ray diffraction studies. (L2)ZnEt was unreactive toward ethanol, but the amide complexes afforded (L2)ZnOEt and (L3)ZnOEt upon reaction with ethanol, which were used in rac-lactide polymerization without isolation. All complexes racemise readily at room temperature and show apparent Cs-symmetry in their NMR spectra. The ethoxide complexes were highly active in lactide polymerization, with (L3)ZnOEt reaching full conversion in 15 min at 0.5 mM catalyst concentration at room temperature. In both cases, introduction of a second donor arm on the central nitrogen introduced a slight bias for isotactic monomer enchainment (Pm = 0.55–0.60), which for (L3)ZnOEt was dependent on catalyst concentration.


 Please wait while we load your content...
Please wait while we load your content...