Insight into the stereoselectivity of TS-1 in epoxidation of cis/trans-2-hexene: a computational study†
Abstract
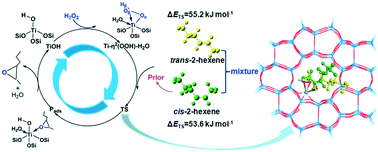
The mechanism of the stereoselectivity for cis/trans-2-hexene epoxidation in TS-1 zeolite with hydrogen peroxide has been investigated using density functional theory with the ONIOM scheme. A cluster model of 136T with Ti(IV) at the T8 site of TS-1 was adopted to mimic the zeolite catalyst. Four different Ti-hydroperoxo intermediates were constructed and optimized. The stability order is Ti-η2(OOH)–H2O–(Hβ)H2O > Ti-η2(OOH)–H2O > Ti-η2(OOH)–(Hβ)H2O > Ti-η2(OOH). The activation energies of cis-2-hexene epoxidation over Ti-η2(OOH), Ti-η2(OOH)–H2O, Ti-η2(OOH)–(Hβ)H2O, and Ti-η2(OOH)–H2O–(Hβ)H2O active centers are 38.6, 53.6, 52.1, and 64.0 kJ mol−1, respectively. The corresponding activation energies for trans-2-hexene epoxidation are 60.1, 55.2, 80.5, and 85.8 kJ mol−1, respectively. cis-2-Hexene had a lower activation energy barrier than trans-2-hexene. The Ti-η2(OOH)–H2O species is likely the real active center; the ratio of the rate constant of the cis-2-hexene to trans-2-hexene epoxidation is 1.7, which agrees nicely with the experimental results. The decisive factor for cis-selectivity depends on the microstructure of the Ti-hydroperoxo intermediate and the confinement effect in TS-1 zeolite. At the transition state, cis-2-hexene is forced to adopt a twisting conformation and embeds suitably in the zeolite cavity under the action of confinement. This leads to increased stability and reduced activation barriers.


 Please wait while we load your content...
Please wait while we load your content...