Impact of pore size, interconnections, and dynamic conductivity on the electrochemistry of vanadium pentoxide in well defined porous structures†
Abstract
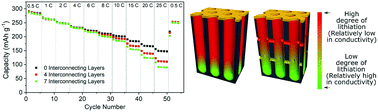
Considering the tortuous, random porous nanostructures existing in many battery electrodes, it is essential to understand electronic and ionic behaviors in such a confined nanoscale porous geometry in which electron and ion transports can change dynamically. Here, we have carefully designed three dimensional (3D) interconnected porous electrode structures and performed experiments to probe how the ion and electron transport is impacted within these controlled geometries. By using anodized aluminum oxide as a template, we were able to fabricate both 1D array electrodes and 3D electrodes with varying numbers of interconnections, utilizing vanadium oxide (V2O5) as the active material. We demonstrate that the inherent properties of the electrode material in combination with the structural properties of the electrodes can both positively and negatively impact electrochemical characteristics. Most notably, electrodes with seven interconnecting layers in their structure had 19.7% less capacity at 25C than electrodes with zero interconnecting layers, demonstrating the negative effect of interconnections combined with poor electronic conductivity of V2O5 upon lithiation beyond one Li insertion. These results indicate that a careful consideration of the material and structural properties is needed for the design of high performance battery systems.


 Please wait while we load your content...
Please wait while we load your content...