Heat capacities and an updated thermodynamic model for the Li–Sn system
Abstract
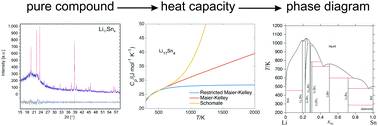
Phase-pure Li17Sn4 and Li7Sn3 intermetallic compounds were synthesized using defined heat treatment procedures in specially designed Ta crucibles. The products were characterized by inductively coupled plasma optical emission spectroscopy (ICP-OES) and powder X-ray diffraction (powder-XRD) techniques to determine their composition and phase purity. The heat capacities of the synthesized compounds were measured via the step method in sealed Ta crucibles using a Setaram C80 Tian-Calvet calorimeter. The experimentally determined heat capacities were then used to develop restricted Maier–Kelley models for each phase and to incorporate these data into the re-optimization of an existing thermodynamic description of the Li–Sn system. The new models for the intermetallic and liquid phases result in improved agreement between the calculated and experimental heat capacity, thermodynamic, electrochemical and phase diagram data. Since the re-optimized Gibbs free energy expressions are based on reliable heat capacity data, the models can now be used to predict emf values in the Li–Sn system at a variety of operation temperatures and active material compositions.


 Please wait while we load your content...
Please wait while we load your content...