Viscous interfacial layer formation causes electroosmotic mobility reversal in monovalent electrolytes†
Abstract
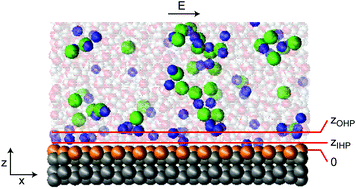
We study the ion density, shear viscosity and electroosmotic mobility of an aqueous monovalent electrolyte at a charged solid surface using molecular dynamics simulations. Upon increasing the surface charge density, ions are displaced first from the diffuse layer to the outer Helmholtz layer, increasing its viscosity, and subsequently to the hydrodynamically stagnant inner Helmholtz layer. The ion redistribution causes both charge inversion and reversal of the electroosmotic mobility. Because of the surface-charge dependent interfacial hydrodynamic properties, however, the charge density of mobility reversal differs from the charge density of charge inversion, depending on the salt concentration and the chemical details of the ions and the surface. Mobility reversal cannot be described by an effective slip boundary condition alone – the spatial dependence of the viscosity is essential.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...