Vibrational spectra of small methylamine clusters accessed by an ab initio anharmonic approach†
Abstract
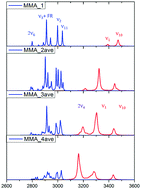
Methylamine (MMA) is one of the simplest amines, and the vibrational spectra of its dimer have recently been obtained experimentally. The vibrational spectra of NH stretch modes were well resolved, but the complex features of the CH3 group could not be fully accounted for even with the assistance of ab initio molecular dynamics (AIMD) with various density functional methods. In this study, we carried out anharmonic vibrational calculations on MMA clusters up to tetramers using MP2/aug-cc-pVDZ to examine vibrational coupling among CH/NH and compute the vibrational spectra of these clusters between 2800 and 3500 cm−1. We found that the main origin of the complexity between 2800 and 3000 cm−1 was caused by Fermi resonance (FR) between the stretching and bending overtones of the CH3 group. This spectral feature becomes simpler in trimers and tetramers. Furthermore, Fermi resonance in the NH2 group is found to be very strong. In the MMA dimer, no noticeable FR features can be found; however, in its trimers and tetramers, the enhancement of hydrogen bond strength due to the cooperative effect will cause the N–H stretching mode to red-shift to revert the energy order of the fundamental of the N–H stretch and overtone of N–H bending between n = 3 and n = 4. Therefore, significant re-distribution of the intensities of the bands at 3200 and 3300 cm−1 should be seen.


 Please wait while we load your content...
Please wait while we load your content...