Alkaline-earth (Be, Mg and Ca) bonds at the origin of huge acidity enhancements†
Abstract
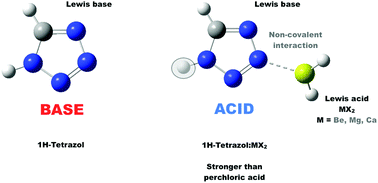
The interaction between alkaline-earth derivatives with the general formula X2M (X = H, F and Cl; M = Be, Mg and Ca) and a set of Lewis bases, including first and second-row hydrides, namely YHn (Y = O, N, F, S, P and Cl) hydrides, as well as other typical cyclic organic bases, such as aniline, 1H-1,2,3-triazole, 1H-tetrazole and phenylphosphine, was investigated using the G4 ab initio composite method. Contrary to what was expected, it was found that the interactions involving Mg and Ca derivatives were not necessarily weaker than those between beryllium bonds. The origin is two-fold: larger deformation of the interacting systems when Be-derivatives are involved and appearance of secondary non-covalent interactions in the formation of some of the Mg- and Ca-containing complexes. Hence, the dissociation of the latter complexes may require higher enthalpies than that of the Be complexes. These deformations are triggered by a significant redistribution of electron density of the two interacting moieties, which also result in dramatic changes in the reactivity of the interacting compounds and in particular in the intrinsic basicity of the Lewis bases investigated, to the point that conventional bases, such as ammonia or aniline, upon complexation with MCl2 (M = Be, Mg and Ca), become stronger Brønsted acids than phosphoric acid, whereas other bases, such as 1H-tetrazole, become stronger acids than perchloric acid.


 Please wait while we load your content...
Please wait while we load your content...