Revealing at the molecular level the role of the surfactant in the enhancement of the thermal properties of the gold nanofluid system used for concentrating solar power
Abstract
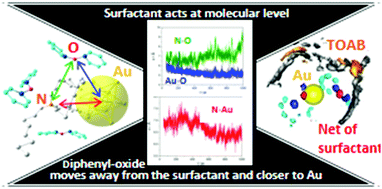
A molecular dynamics study based on gold nanofluids performed with and without the presence of tetraoctylammonium halide as a surfactant in a base fluid is presented. The base fluid consisting of a mixture of biphenyl and diphenyl oxide is used in concentrating solar power (CSP) plants. The radial distribution functions (RDFs) and spatial distribution functions (SDFs) were analysed with the temperature. Theoretical results indicate that the surfactant acts as a kind of net around the nanoparticle that plays an active role in enhancing the thermal properties of the gold nanofluid system. A greater lability of the base fluid–surfactant interactions than the base fluid–gold nanoparticle interactions is observed. At lower temperatures, there is an inner layer around the gold nanoparticle with two surfactant molecules close to the metal. At a higher temperature a ratio of gold nanoparticles : diphenyl oxide molecules of 1 : 4 is maintained in the inner layer for the systems with and without the presence of a surfactant. At the highest temperatures, the presence of the surfactant in a second shell impedes the approximation of the fifth diphenyl oxide molecule. Thus, the surfactant affects the macroscopic properties of the gold nanofluid system at the molecular level.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...