Four new MOFs based on an imidazole-containing ligand and multicarboxylates: syntheses, structures and sorption properties†
Abstract
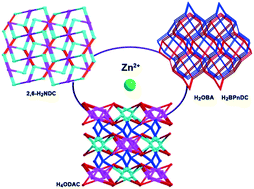
Four new complexes have been synthesized by solvothermal reactions of an imidazole-containing ligand 3,3′,5,5′-tetra(1H-imidazol-1-yl)-1,1′-biphenyl (L) with corresponding metal salts via the alternation of auxiliary multicarboxylates, [Zn2(L)(2,6-NDC)(OAc)2]·2H2O (1), [Zn2(L)(BPnDC)2]·6H2O (2), [Co2(L)(OBA)2]·8H2O (3) and [Zn4(L)2(ODAC)2]·2MeCN·5H2O (4) (2,6-H2NDC = 2,6-naphthalic acid, H2BPnDC = 4,4′-carbonyldibenzoic acid, H2OBA = 4,4′-oxidibenzoic acid, and H4ODAC = 3,3′,4,4′-oxidibenzene tetracarboxylic acid). The complexes were characterized by single crystal and powder X-ray diffractions, IR, elemental and thermogravimetric analysis. All of them possess different three-dimensional (3D) frameworks based on M(II)–L two-dimensional layers, which are further connected by auxiliary dicarboxylates with distinct bridging-angles and coordination numbers to obtain the final frameworks. Complex 1 is a (3,4)-connected 3D framework with gwg topology, while 2 and 3 are isostructures with a (4,4)-connected 2-fold interpenetrating 3D net. In the case of 4, it possesses a (4,4,4,4)-connected 3D architecture with a new topology. The desolvated samples 2′ and 3′ have adsorption capacities of H2O/MeOH/EtOH, while sample 4′ has remarkable selective adsorption for H2O/MeOH over EtOH.


 Please wait while we load your content...
Please wait while we load your content...