Simple preparation of potassium sulfate nanoparticles
Abstract
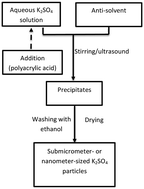
Water-soluble salts are widely used in the synthesis of porous materials, hollow materials, and nanostructured materials as templates or isolation media. However, there are some restrictions on their application, as it is difficult to prepare nanoscale water-soluble salt particles. In this study, potassium sulfate nanoparticles were prepared by anti-solvent precipitation, and the effects of parameters such as the initial concentration, mixing order, and surfactant addition on the particle size were investigated. Polyacrylic acid was found to promote nucleation, and the size of the particles obtained in its presence was reduced significantly. As the amount of polyacrylic acid increased, the particle size decreased, such that the average particle size could be controlled within the range of 10–100 nm by adjusting the amount of additive. This method can be used to prepare potassium sulfate nanoparticles on a large scale; further, the obtained nanoparticles should be suitable as sacrificial template materials for preparing nanoporous materials, hollow nanomaterials, and other nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...