Novel Cu(ii) complexes with NNO-Schiff base-like ligands – structures and magnetic properties†
Abstract
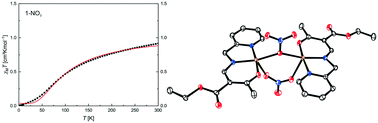
We present a series of six new tridentate Schiff base-like ligands, derived from 2-picolylamine, providing an NNO coordination sphere. Their corresponding Cu(II) complexes were synthesised with a range of varying counter anions (OAc−, NO3−, Cl−, I−, NCS−, and N3−). The results from single X-ray structure analyses of four ligands and 22 Cu(II) complexes are presented. The majority of the complexes crystallised as dimers with the anion bridging the Cu(II) centres in a μ-fashion; depending on the substituents at the ligand and the counter ion the formation of coordination polymers or mononuclear complexes is also possible. Temperature dependent magnetic measurements revealed that the exchange interactions between the Cu(II) centres depend on the nature of the bridging ligand (axial/equatorial), the Cu–X–Cu angle, and the distortion between a square pyramidal and a trigonal bipyramidal coordination sphere, explicable by assuming a superexchange.


 Please wait while we load your content...
Please wait while we load your content...