Controlled self-assembly of π-stacked/H-bonded 1D crystal structures from polyfluorinated arylamines and 18-crown-6 (2 : 1). Associate vs. co-former fluorescence properties†
Abstract
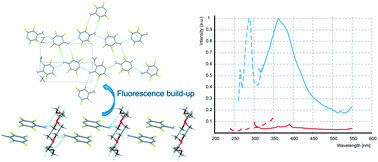
Supramolecular 1D assemblies supported by N–H⋯Ocr and π-stacking interactions were designed and synthesized using polyfluorinated arylamines, differing in the nature of the aromatic ring (benzene, naphthalene, pyridine) and para-substituents (H, F, CN, CF3), and 18-crown-6 ether. Co-crystal structures and intermolecular interactions were studied in detail using X-ray analysis and compared with those in individual arylamine crystals. In the co-crystals, all relatively weak H-bonds N–H⋯X (X = F, Nhet, Ncyano) are replaced by the stronger bond N–H⋯Ocrown and π-stacking in pairs of the arylamine molecules is realized. Depending on the arylamine structure, a parallel displaced, rotated, or slipped stack geometry occurs. Using DSC, phase transitions in the co-crystals were determined and the thermal characteristics were found to be reproduced in the melting-crystallization cycles, which indicates the regeneration of the crystal structure. For a number of compounds, there are significant changes in the fluorescence characteristics, in terms of both intensity and wavelength, due to the formation of associates with 18-crown-6: aggregation-induced emission in the pentafluoroaniline associate, a large shift of λem in the 4-cyanotetrafluoroaniline associate, and aggregation-caused quenching in the 4-aminotetrafluoropyridine associate. Crystal structure analysis reveals that π-stacking interactions between arylamines play a key role in the modulation of the solid-state fluorescence properties. The revealed effect of fluorescence build-up/quenching shows these types of supramolecular associates to be promising solid-state chemosensors.


 Please wait while we load your content...
Please wait while we load your content...