Oxidative coupling of enolates using memory of chirality: an original enantioselective synthesis of quaternary α-amino acid derivatives†
Abstract
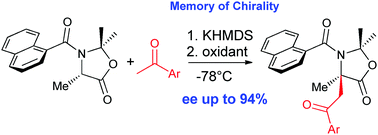
We describe here the first enantioselective oxidative heterocoupling of enolates. Our strategy relies on the memory of chirality concept and allows the stereocontrolled formation of quaternary centres on α-amino acid derivatives with an enantiomeric excess of up to 94%.


 Please wait while we load your content...
Please wait while we load your content...