Tuning of protease resistance in oligopeptides through N-alkylation†
Abstract
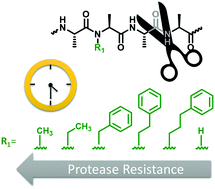
N-Methylation of amino acids is an effective way to create protease resistance in both natural and synthetic peptides. However, alkyl substituents other than N-methyl have not been extensively studied. Here, we prepare and examine a series of N-substituted peptides in which the size and length of the alkyl group is modulated. These design insights provide a unique and modular handle for tuning proteolysis in oligopeptides.


 Please wait while we load your content...
Please wait while we load your content...
