Single-molecule force-unfolding of titin I27 reveals a correlation between the size of the surrounding anions and its mechanical stability†
Abstract
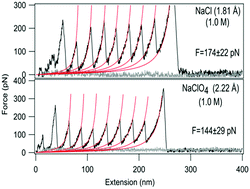
Each cellular protein is surrounded by a biochemical milieu that affects its stability and the associated function. The role of this surrounding milieu in the proteins’ mechanical stability remains largely unexplored. Herein, we report an as yet unknown correlation between the size of the surrounding anions and the mechanical stability of a protein. Using single-molecule force spectroscopy of the 27th domain (I27) of human cardiac muscle protein titin, we show that the average unfolding force of the protein decreases with increase in the ionic radii of the surrounding anions in the order Cl− > Br− > NO3− > I− > SO42− ≈ ClO4−, indicating an inverse correlation between anion size and the mechanical stability of I27. The destabilizing effect was attributed to the combined effect of increase in the unfolding rate constant and unfolding distance upon incubation with the anion. These findings reveal that anion size can significantly affect the mechanical resistance of proteins and thus could be a convenient and promising tool for regulating the mechanical stability of proteins.


 Please wait while we load your content...
Please wait while we load your content...