Human histone demethylase KDM6B can catalyse sequential oxidations†
Abstract
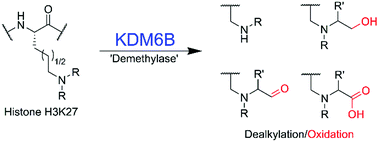
Jumonji domain-containing demethylases (JmjC-KDMs) catalyse demethylation of Nε-methylated lysines on histones and play important roles in gene regulation. We report selectivity studies on KDM6B (JMJD3), a disease-relevant JmjC-KDM, using synthetic lysine analogues. The results unexpectedly reveal that KDM6B accepts multiple Nε-alkylated lysine analogues, forming alcohol, aldehyde and carboxylic acid products.


 Please wait while we load your content...
Please wait while we load your content...