Controlled photorelease of alkynoic acids and their decarboxylative deprotection for copper-catalyzed azide/alkyne cycloaddition†
Abstract
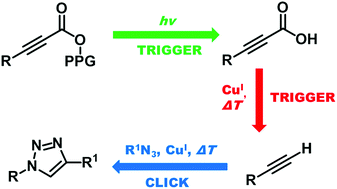
A controlled photorelease of alkynoic acids from the meso-methyl BODIPY photoremovable protecting group facilitates their subsequent efficient decarboxylation to give terminal alkynes for a CuI-catalyzed azide/alkyne cycloaddition. The quantum efficiencies of the photochemical step and the kinetics of the click reaction step are reported.


 Please wait while we load your content...
Please wait while we load your content...