Electrostatic interaction mechanism based synthesis of a Z-scheme BiOI–CdS photocatalyst for selective and sensitive detection of Cu2+†
Abstract
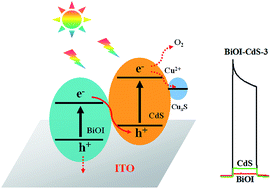
A direct Z-scheme BiOI–CdS photocatalyst has been successfully synthesized by an electrostatic interaction mechanism and used for selective photoelectrochemical sensing of Cu2+. The BiOI–CdS photocatalyst shows higher photocatalytic activity and photoelectrochemical performance than pure CdS and BiOI. The photocurrent intensity generated by the BiOI–CdS-3 electrode is about 62 and 10 times of those induced by BiOI and CdS under visible-light irradiation, respectively. The enhanced photocatalytic activity is attributed to the formation of a hierarchical direct Z-scheme BiOI–CdS photocatalyst and its high Brunauer–Emmett–Teller (BET) specific surface area, which benefit the efficient spatial separation of charge and capture of visible-light. Moreover, a photoelectrochemical sensor is developed based on the selective replacement reaction between Cu2+ and CdS. The photoelectrochemical sensor is easily fabricated and presents good selectivity, acceptable detection range (0.1–100 μM) and detection limit (0.02 μM). It has been applied to the detection of Cu2+ ions in drinking water with a satisfactory result.


 Please wait while we load your content...
Please wait while we load your content...