Investigation into the stability of Li metal anodes in Li–O2 batteries with a redox mediator†
Abstract
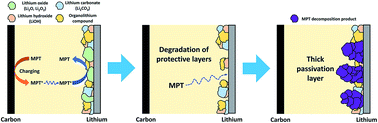
The recent introduction of redox mediators as soluble catalysts in electrolytes has aimed at overcoming the spatial constraint of solid catalysts in lithium–oxygen (Li–O2) batteries. The redox mediators show promise for improving electrochemical performance, for example, by decreasing the overpotential for oxygen evolution reactions. However, their catalytic activity degrades after a certain number of cycles, leading to an increase in overpotential with increasing cycle number. The shuttle effect of redox mediators has been considered to be associated with the failure of Li–O2 batteries containing the redox mediators, but, this effect does not fully explain the capacity fading observed with Li–O2 batteries. In this study, it is demonstrated that the failure of these types of Li–O2 cells occurs because of an irreversible decomposition of the redox mediators on Li metal, which both degrades Li metal and depletes the redox mediators. We also show that this decomposition occurs even though oxygen dissolved in the electrolytes forms stable protective layers of lithium oxides on the Li surface. One model redox mediator, 10-methylphenothiazine, is examined in a case study to clarify the failure mechanism of Li–O2 batteries.


 Please wait while we load your content...
Please wait while we load your content...