Preparation of MgO nanocrystals and catalytic mechanism on phenol ozonation
Abstract
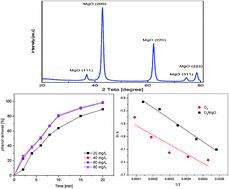
This research aims to clarify the role of magnesium oxide as a catalyst in the catalytic ozonation process. Nano-sized magnesium oxide was prepared by the sol–gel method and characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). The catalytic performance of magnesium oxide was tested for the removal of phenol. The effect of initial pH, MgO nanocrystal amount, radical scavenger (t-butanol) and Rct was investigated to understand the catalytic ozonation mechanism of magnesium oxide with ozone. Experimental results illustrated that nano-sized magnesium oxide presented significant performance for the ozonation and catalyzed the removal of phenol from aqueous solution by ozonation with a radical pathway involving hydroxyl radicals, which was due to the activity of surface basic groups from magnesium oxide where the conversion of ozone to hydroxyl radicals occurred. Fourier transform infrared spectroscopy (FT-IR) and isoelectric point (IEP) analysis was applied to analyze the surface properties of the prepared nano-magnesium oxide. Activation energy (Ea) was calculated based on the Arrhenius principle equation. It reveals that phenol could enhance the density of surface hydroxyl groups and the introduction of magnesium oxide into the ozonation system does not alter the activation energy. Therefore, the prepared powder was found to be an efficient and promising catalyst for ozonation.


 Please wait while we load your content...
Please wait while we load your content...