N,N-Dimethylpyridin-4-amine (DMAP) based ionic liquids: evaluation of physical properties via molecular dynamics simulations and application as a catalyst for Fisher indole and 1H-tetrazole synthesis†
Abstract
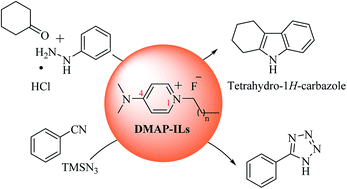
The last few decades have seen a rapid increase in the use of ionic liquids (ILs) as a green alternative to traditional solvents in organic synthesis. The use of ILs as catalysts has also increased in recent years. Herein we the report synthesis of new N,N-dimethylpyridin-4-amine (DMAP) based ionic liquids (ILs) as new and efficient catalysts for the facile synthesis of indoles (via Fischer indole synthesis), and 1H-tetrazoles (via click chemistry). The method is environmentally friendly, requiring only minimum catalyst loading (0.2 equiv. for Fischer indole synthesis). In the case of 1H-tetrazole formation (via click chemistry), the reaction was carried out under a solvent free environment. Moreover, thermal studies (TGA, DTG and DSC) of DMAP-ILs (2, 3) have also been carried out to elicit their stability for temperature dependent reactions. Application of molecular dynamics simulations provided valuable insights into the structural and transport properties of these ionic liquids. An MP2 method was applied to evaluate the stability of the compound via binding energy calculations.


 Please wait while we load your content...
Please wait while we load your content...