Acid-assisted hydrothermal synthesis of red fluorescent carbon dots for sensitive detection of Fe(iii)†
Abstract
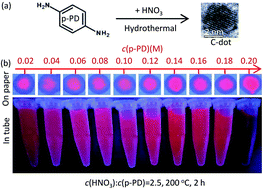
Red-emitting carbon dots (C-dots) were synthesized from p-phenylenediamine (p-PD) aqueous solution with nitric acid (HNO3) assistance by hydrothermal reaction at 200 °C for 2 h. p-PD aqueous solution can be transferred to C-dots (or poly(p-PD)) with (or without) the addition of acid. Different acid systems, such as HNO3, H3PO4 and HF, can directly synthesize red-emitting C-dots, and the fluorescence can be enhanced by increasing the strength of acids. N-CDs, 3.46 nm-average-sized C-dots, prepared in dilute HNO3, have a quantum yield of 15.8% with unique, excitation-wavelength independent emission in the red region (600 and 680 nm) and a broad visible excitation band. Carboxyl, ester and hydroxyl groups on the C-dots surface directly lead to red emission. N-CDs have a certain selective specificity for Fe3+ detection and the linear range is 10–300 nmol L−1 with a limit of determination of 1.9 nmol L−1.


 Please wait while we load your content...
Please wait while we load your content...