The effects of central metals on ammonia sensing of metallophthalocyanines covalently bonded to graphene oxide hybrids†
Abstract
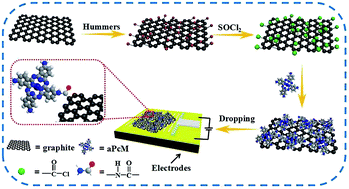
Cost-efficient, highly sensitive, selective and stable sensing materials play a key role in developing NH3 sensors. Herein, a series of tetra-β-aminephthalocyanines metal(II) (aPcMs M = Cu, Ni, Co, Fe) have been successfully covalently bonded on the surface of graphene oxide (GO) by a facile amidation reaction. The obtained aPcM–GO hybrids display good dispersibility, which is beneficial to construct uniform sensing devices. The aPcM–GO sensors exhibit excellent sensing performance in terms of sensitivity, reversibility, reproducibility, selectivity and stability, especially the aPcCo–GO sensor which exhibited a response of about 11.6% (50 ppm), a limit of detection as low as 800 ppb, and a recovery time of about as fast as 350 s at room temperature. The enhanced NH3-sensing performance is mainly due to the synergistic effect between aPcM and GO, e.g. the stronger adsorption interaction of aPcM with NH3, the high electrical conductivity of GO, and the fast charge transfer between aPcM and GO. By comparison, the response of aPcM–GO hybrids to ammonia decreases gradually in the following order of Co > Cu > Ni > Fe ≫ GO, indicating that the central metals play a critical role in gas sensitivity toward NH3, which is further confirmed by first-principle density functional theory.


 Please wait while we load your content...
Please wait while we load your content...