Efficient removal of Pb(ii) ions using manganese oxides: the role of crystal structure
Abstract
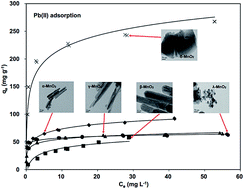
Manganese oxides have been proven to be promising adsorbents to capture Pb(II) from wastewaters. In nature, MnO2 can be found in different crystalline structures, while the effect of crystal structure on their adsorption performance remains unclear. In this study, five manganese oxides with different crystallographic phases, α-, β-, γ-, δ-, and λ-MnO2 were prepared and characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), N2 adsorption–desorption, Fourier transform infrared (FT-IR) spectroscopy and zeta potential measurements. The adsorptive removal of aqueous Pb(II) was investigated using these manganese oxides as adsorbents. The results showed that the adsorption capacities of manganese oxides for Pb(II) varied with BET surface area and crystalline structure, following the order of δ-MnO2 > α-MnO2 > λ-MnO2 > γ-MnO2 > β-MnO2. δ-MnO2 displayed the highest capacity for Pb(II), and the adsorption was scarcely influenced by the presence of the coexisting Na+ cation. The surface complexation model was used to describe the Pb(II) adsorption on the MnO2 adsorbents. In a column adsorption test δ-MnO2 was capable of continuously treating 25 000 bed volumes synthetic wastewater stream with an influent concentration of 20 mg L−1 Pb(II) and an effluent concentration below 0.5 mg L−1. This work indicates that δ-MnO2 has great potential to be used as an effective adsorbent for Pb(II) removal.


 Please wait while we load your content...
Please wait while we load your content...