Improved performance of HZSM-5 for the ethylbenzene/xylene isomerization reaction under industrial operating conditions
Abstract
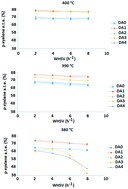
A mild, specific one-step dealumination of ZSM-5 is reported for the first time that significantly increases para xylene selectivity and ethyl benzene conversion by disproportionation in the xylene isomerization reaction of xylene mixtures/ethyl benzene feeds under industrial conditions, i.e., 380 °C, 390 °C and 400 °C, at a pressure of 8 bar, weight hourly space velocity (WHSV) values of 2 to 8 h−1, and a H2/HC ratio of 4. Oxalic acid and ammonium hexafluorosilicate (AHFS) were used as dealuminating agents. Mild increases of the Si/Al molar ratio by one unit using oxalic acid or by 10 units using AHFS resulted in a more than 10% increase in para xylene when approaching equilibrium at 400 °C and WHSV = 2 h−1. However, the oxalic acid-treated sample increased ethylbenzene conversion by more than 30% with respect to the parent zeolite under the applied operating conditions. The AHFS-treated samples exhibited decreases in ethylbenzene conversion irrespective of the extent of dealumination.


 Please wait while we load your content...
Please wait while we load your content...