New endeavours involving the cooperative behaviour of TMAO and urea towards the globular state of poly(N-isopropylacrylamide)†
Abstract
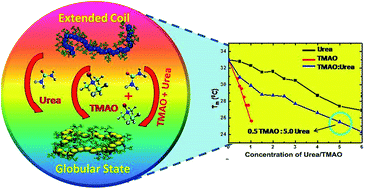
Studies have provided evidence for the destruction of the hydrogen bonds of poly(N-isopropylacrylamide) (PNIPAM) in the presence of osmolytes such as trimethylamine N-oxide (TMAO) and urea. Herein, we explore the effect of a mixed osmolytic environment on PNIPAM, which has thus far been an unanswered question in the literature, and we show the ease with which the addition of co-solvents like urea and TMAO individually, and as a mixture, result in the quick formation of the globular state of PNIPAM. The investigation of the globular or collapsed state of aqueous PNIPAM solution in the presence of co-solvents is exploited using various biophysical techniques like UV-visible spectroscopy, fluorescence spectroscopy, dynamic light scattering studies (DLS) and Fourier transform infrared spectroscopy (FTIR). The decrease in the lower critical solution temperature (LCST) with the addition of osmolytes and their mixed environment may be due to the different types of interactions (direct or indirect) with the hydrated, extended, coiled and globular PNIPAM states. The extent of hydrogen bonding and polarization of these additives is the major driving force for the collapsed conformation of PNIPAM. TMAO is a better inducer for the collapsed state of PNIPAM than urea, and their mixture shows a drastic decrease in the LCST of PNIPAM, because of the LCST decrease in both osmolytes individually, but the limited number of binding sites of PNIPAM is the main reason the mixture of urea and TMAO is not able to show an additive effect on PNIPAM agglomeration. Varying ratios of TMAO and urea are compared for a clear understanding of their behavior toward PNIPAM. Their mixed environment is a very good inducer of the globular form, compared to individual urea at higher concentration. The collapsed state of the polymer may provide sites or domains for drug encapsulation and release at the target by changing its conformation.


 Please wait while we load your content...
Please wait while we load your content...