Synthesis and characterisation of cerium(iv)-incorporated hydrous iron(iii) oxide as an adsorbent for fluoride removal from water†
Abstract
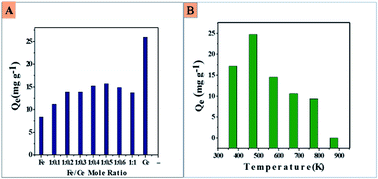
Surface-altered hydrous iron(III) oxide incorporating cerium(IV) (CIHFO) was prepared and characterised via modern analytical tools for applications in fluoride removal from groundwater. The material with a Fe : Ce ratio of 1.0 : 0.5 (mol : mol) calcined at 473 K shows 24.8 ± 0.5 mg F− g−1 adsorption capacity at pH 5.0–7.0 from a solution with a concentration of 15.0 mg L−1; the material was established to be microcrystalline (∼5 nm) with a 140.711 m2 g−1 surface area, irregular surface morphology and porous structure. The time-dependent fluoride adsorption capacities of CIHFO at 293, 303 and 313 K are well described by the pseudo-first order, pseudo-second order and Weber–Morris kinetic models, respectively. The adsorption reaction occurs via a film/boundary layer diffusion process. The very low Arrhenius activation energy (Ea = 0.026 kJ mol−1) indicates the high feasibility of fluoride adsorption over CIHFO. The equilibrium data fit better with the Freundlich and Redlich–Peterson (g < 1.0) isotherms than with the Langmuir isotherm, which suggests multilayer adsorption. The values of the Freundlich parameters, n = 3.10, 4.47 and 7.57 and KF = 8.58, 10.88 and 11.25 at 293, 303 and 313 K, respectively, indicate high affinity for fluoride. Thermodynamic analysis of the reaction equilibrium shows that the reaction is highly exothermic (ΔH0 = −25.924 and −36.279 kJ mol−1 for Ci = 25.0 and 35.0 mg L−1), whereas the negative ΔG0 values indicate the spontaneous nature of the reaction. The fluoride adsorption over CIHFO occurs via ion-exchange that progresses to chemisorption. The presence of sulphate shows an adverse influence on fluoride adsorption by CIHFO, and the fluoride level of 2.4 g per L groundwater (9.05 mg F L−1) can be reduced below the permissible value.


 Please wait while we load your content...
Please wait while we load your content...