Aqueous MCRs of quaternary ammoniums, N-substituted formamides and sodium disulfide towards aryl thioamides†
Abstract
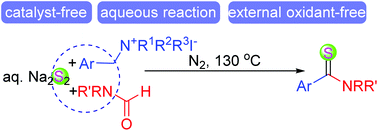
A three-component reaction of quaternary ammonium salts, N-substituted formamides and aqueous sodium disulfide was developed, leading to aryl/heteroaryl thioamides in moderate to good yields with good functional group compatibility. This procedure was featured with the combination of three reaction partners, rendering an important complementary to the previously reported methods for the synthesis of aryl thioamides, and was beneficial to the chemistry of functional group transformations. No external oxidant was required in this procedure.


 Please wait while we load your content...
Please wait while we load your content...