New reactivity of ethynyl benziodoxolone: modulating iron-catalyzed dehydration of propargyl alcohols†
Abstract
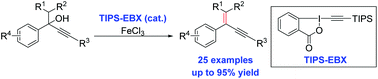
A practical method for dehydration of alcohols, mainly propargyl alcohols, promoted by silyl-EBX is reported, which represents the first example of using only a catalytic amount of silyl-EBX since its discovery. A broad range of aryl propargyl alcohols proceeded smoothly and gave the dehydration products in good to excellent yields. Preliminary mechanistic studies ruled out the radical pathways and supported an E1 process for this reaction. The carbon–carbon triple bond and the lactone moiety within the EBX molecule may have played an important role in the reaction.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2016

 Please wait while we load your content...
Please wait while we load your content...