A photo-responsive polymeric azopyridine ligand with metal-complexation sensitivity: application to coordination equilibrium studies on the polymer complexes of a cobalt(ii) Schiff base†
Abstract
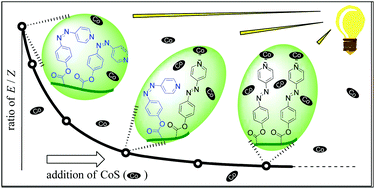
In a solution of the copolymer, P(EHMA-APMA), consisting of ethylhexyl methacrylate (EHMA) and the photo-responsive azopyridine methacrylate (APMA), the E form of APMA showed photo-reversible isomerization to the Z form upon UV irradiation. After addition of the cobalt(II) Schiff base (CoS), the copolymer complex, P(EHMA-(E)-APMA)-CoS, was produced, and the (E)-APMA moieties coordinated to CoS no longer underwent photo-induced isomerization. The complexation-sensitive isomerization of (E)-APMA had a large extinction coefficient and the apparent equilibrium constant (Kapp) for the coordination reaction of (E)-APMA with CoS in a dilute solution was calculated to be 4.4 × 104 M−1 ([APMA moiety] = 0.05 mM) and 1.0 × 105 M−1 ([APMA moiety] = 0.003 mM) at 25 °C. The resulting concentration-dependent large Kapp values were due mainly to a micro-heterogeneous solution environment, consisting of a micro-suspension with a large solvent phase and a small polymer-domain phase caused by the extreme dilution, in addition to the thermodynamic stabilization of the five-coordinate CoS via the local concentration effect of the coordinating polymeric APMA ligands located only in the polymer domain.


 Please wait while we load your content...
Please wait while we load your content...