Copper catalyzed oxidative homocoupling of terminal alkynes to 1,3-diynes: a Cu3(BTC)2 MOF as an efficient and ligand free catalyst for Glaser–Hay coupling†
Abstract
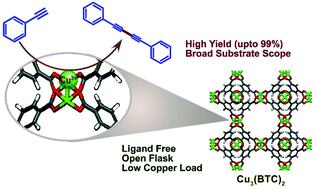
A straightforward and efficient method has been demonstrated for the oxidative coupling of terminal alkynes using a simple Cu3(BTC)2-metal organic framework as a sustainable heterogeneous copper catalyst. A series of symmetrical 1,3-diynes bearing diverse functional groups have been synthesized in moderate to excellent yields via a Cu3(BTC)2 catalyzed Glaser–Hay reaction. The presence of the coordinatively unsaturated open CuII sites in Cu3(BTC)2 catalyzes the homocoupling in the presence of air, as an environment friendly oxidant without the use of external oxidants, ligands or any additives. The present methodology avoids stoichiometric reagents and harsher or special reaction conditions, and shows good functional group tolerance. The as-prepared catalyst could be separated easily by simple filtration and reused several times without any notable loss in activity. The hot filtration test has investigated the true heterogeneity of the catalyst. Additionally, the powder X-ray diffraction pattern of the reused catalyst revealed the high stability of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...